Advanced Research and Quality Control
Each product undergoes rigorous testing and clinical trials to ensure its safety and efficacy before receiving approval from regulatory authorities like the FDA.
Advanced Research and Quality Control
Each product undergoes rigorous testing and clinical trials to ensure its safety and efficacy before receiving approval from regulatory authorities like the FDA.
Cardiorenal
Products
We specialize in launching cardiovascular and renal therapies, putting patients first in everything we do. Our expertise spans commercialization and navigating the complexities of healthcare regulations, ensuring breakthrough treatments efficiently reach the patients who need them most.
Starting with the problem, we develop tailored solutions that align with actual market needs. Focusing on chronic conditions and cost-effectiveness, our novel approach enhances patient care and accelerates early commercial success.
FUROSCIX®
(furosemide injection) 80mg/10mL for Subcutaneous Use.
Preclinical
Phase 1
Phase 2
Phase 3
Phase 4
Supports fluid management in chronic heart failure and chronic kidney disease
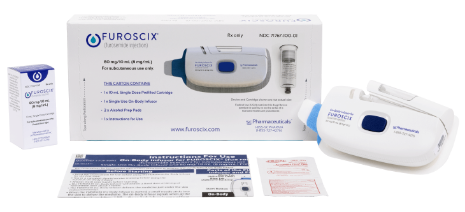
INDICATION
FUROSCIX® (furosemide injection), 80 mg/10 mL for subcutaneous use is indicated for the treatment of edema (i.e., congestion, fluid overload, or hypervolemia) in adult patients with chronic heart failure or chronic kidney disease (CKD), including the nephrotic syndrome.
IMPORTANT SAFETY INFORMATION
FUROSCIX is contraindicated in patients with anuria and in patients with a history of hypersensitivity to furosemide, any component of the FUROSCIX formulation, or medical adhesives.
Furosemide may cause fluid, electrolyte, and metabolic abnormalities, particularly in patients receiving higher doses, patients with inadequate oral electrolyte intake, and in elderly patients. Serum electrolytes, CO2, BUN, creatinine, glucose, and uric acid should be monitored frequently during furosemide therapy.
Excessive diuresis may cause dehydration and blood volume reduction with circulatory collapse and possibly vascular thrombosis and embolism, particularly in elderly patients.
Furosemide can cause dehydration and azotemia. If increasing azotemia and oliguria occur during treatment of severe progressive renal disease, discontinue furosemide.
Cases of tinnitus and reversible or irreversible hearing impairment and deafness have been reported with furosemide. Reports usually indicate that furosemide ototoxicity is associated with rapid injection, severe renal impairment, the use of higher than recommended doses, hypoproteinemia or concomitant therapy with aminoglycoside antibiotics, ethacrynic acid, or other ototoxic drugs.
In patients with severe symptoms of urinary retention (because of bladder emptying disorders, prostatic hyperplasia, urethral narrowing), the administration of furosemide can cause acute urinary retention related to increased production and retention of urine. These patients require careful monitoring, especially during the initial stages of treatment.
Contact with water or other fluids and certain patient movements during treatment may cause the On-body Infusor to prematurely terminate infusion. Ensure patients can detect and respond to alarms.
The most common adverse reactions with FUROSCIX administration in clinical trials were site and skin reactions including erythema, bruising, edema, and injection site pain.
Please see the full Prescribing Information and Instructions for Use.
Advancing Cardiovascular and Renal Therapies with Clinical Precision
We focus on bringing cardiovascular and renal therapies to market, always putting patients first. Our solutions are designed to address real market needs, targeting chronic conditions with a cost-effective approach that enhances patient care and drives early success.