Dedicated to Improving Patient Lives
At the forefront of innovation, we develop advanced treatments aimed at transforming patient care and improving global health outcomes.
Dedicated to Improving Patient Lives
At the forefront of innovation, we develop advanced treatments aimed at
transforming patient care and improving global health outcomes.
About scPharma
Our Company

Who We Are
scPharmaceuticals is a commercial-stage pharmaceutical company focused on redefining cardiorenal care with integrated treatments designed to address the unmet needs of patients. By developing accessible healthcare solutions, we are dedicated to improving health outcomes and quality of life for those we serve.

scPharmaceuticals is Driving Meaningful Change
We have developed a comprehensive approach to managing complex conditions. Leveraging our end-to-end commercial expertise, strong industry relationships, and a specialty care model, we work closely with patients, caregivers, healthcare professionals, regulators, payers, late-stage R&D companies, and academic institutions to advance cardiorenal care. Our collaborative efforts are focused on addressing today’s healthcare challenges and driving progress to solve the most pressing unmet patient needs.


Our Story
In 2013, scPharmaceuticals was founded with a vision to transform patient lives by advancing outpatient care and reduce healthcare costs. In October 2022, we launched our first product to market supporting the treatment of congestion due to fluid overload in adult patients with chronic heart failure, a critical consequence that results in symptoms like weight gain weight gain, swelling, fatigue, and difficulty breathing. This condition affects approximately 6.7 million adults in the United States, resulting in 1 to 2 million hospitalizations and over 85,000 deaths annually.
Bozkurt, Biykem, et al. “HF STATS 2024: Heart Failure Epidemiology and Outcomes Statistics an Updated 2024 Report from the Heart Failure Society of America.” Journal of Cardiac Failure, vol. 31, no. 1, 1 Sept. 2024, www.sciencedirect.com/science/article/pii/S107191642400232X?via%3Dihub, https://doi.org/10.1016/j.cardfail.2024.07.001.
Our Future
As a leader in patient-focused healthcare, scPharmaceuticals continues to expand our offerings with a commitment to delivering cost-effective and specialized solutions. We believe that addressing unmet needs in the cardiorenal space will transform patient care, providing a more accessible and effective approach to treatment.
scPharmaceuticals
Our History
- 2020
- 2021
- 2022
- 2023
- 2024
- 2025
-
2020
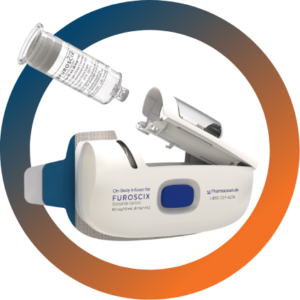
FUROSCIX Resubmission
- FUROSCIX NDA Resubmission
- Receives Complete Response Letter from FDA for FUROSCIX®
-
2021

FREEDOM-HF Results
- Positive Top-Line Results from FREEDOM-HF Study
-
2022

FUROSCIX Approval and Financing
- Resubmission of FUROSCIX® (furosemide injection) New Drug Application
- Positive Results from the AT HOME-HF Phase 2 Pilot Study in Heart Failure
- FDA Approval of FUROSCIX® (furosemide injection)
- $100 Million Debt Financing Agreement with Oaktree
-
2023

FUROSCIX Launch
- Launch and Commercial Availability of FUROSCIX® (furosemide injection)
- Positive Type C Meeting Feedback from FDA on Potential Heart Failure Class IV Indication Expansion
-
2024

Indication Expansion Progress
- First Participant Enrolled in Pivotal Pharmacokinetic Study of FUROSCIX Auto-Injector (furosemide 80mg/mL) Injection
- Filing Acceptance of Supplemental New Drug Application (sNDA) Seeking to Expand FUROSCIX Indication to Include Chronic Kidney Disease
- Positive Topline Study Results for SCP-111 (Furosemide 80 mg/1 mL) Autoinjector
- FDA Approval of Supplemental New Drug Application Expanding the FUROSCIX Indication in Heart Failure
- Financings Totaling Up to $125 Million with Perceptive Advisors
-
2025

Continued FUROSCIX Growth
- Chronic Kidney Disease indication FDA approved on March 6, 2025
