Empowering Health, Transforming Lives
We are focused on advancing cardiorenal innovation through integrated treatments that address unmet patient needs
Empowering Health, Transforming Lives
We are focused on advancing cardiorenal innovation through integrated treatments that address unmet patient needs
Patient-Driven
Cardiorenal Care
The scPharma Difference
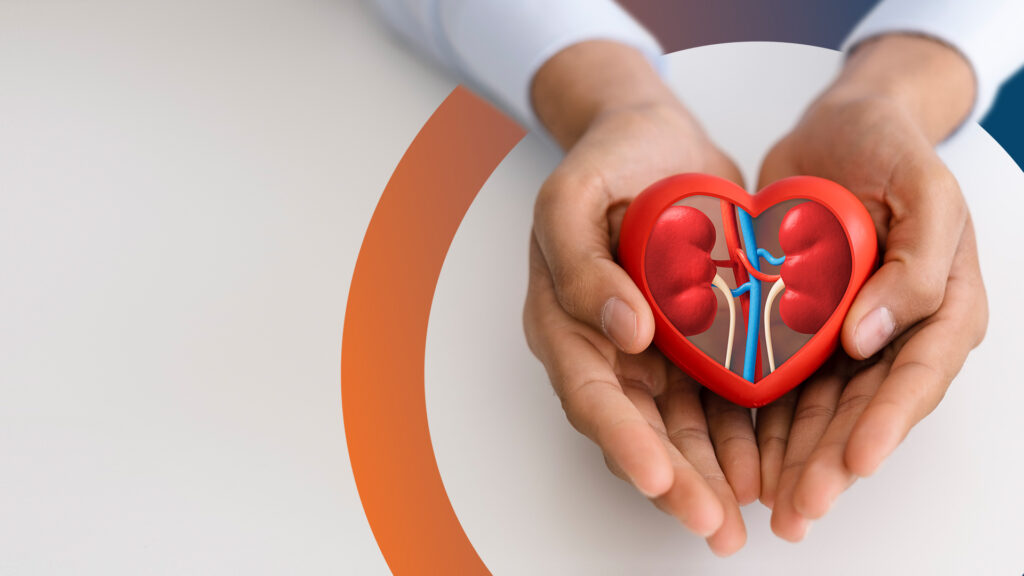
Pioneering Care for the Heart and Kidney Connection
scPharmaceuticals is committed to revolutionizing cardiovascular and renal care through patient-centric approaches tailored to enhance outcomes, reduce burden, and increase quality of life.
Patient-Driven
Cardiorenal Care
The scPharma Difference
Pioneering Care for the Heart and Kidney Connection
scPharmaceuticals is committed to revolutionizing cardiovascular and renal care through patient-centric approaches tailored to enhance outcomes, reduce burden, and increase quality of life.
Pharmaceutical Development, Drug-Device Combinations,
and Product Commercialization Expertise
Integrated Expertise
Leading the Way in Value-Based Treatments for Heart Failure and Chronic Kidney Disease
Our focus on integrated, value-driven care positions us as a trusted leader in the industry, committed to improving patient lives and redefining healthcare standards.

Pharmaceutical Development, Drug-Device Combinations,
and Product Commercialization Expertise
Integrated Expertise
Leading the Way in Value-Based Treatments for Heart Failure and Chronic Kidney Disease
Our focus on integrated, value-driven care positions us as a trusted leader in the industry, committed to improving patient lives and redefining healthcare standards.
scPharmaceuticals
Cardiorenal Journey
- 2020
- 2021
- 2022
- 2023
- 2024
- 2025
-
2020
FUROSCIX Resubmission
- FUROSCIX NDA Resubmission
- Receives Complete Response Letter from FDA for FUROSCIX®
-
2021

FREEDOM-HF Results
- Positive Top-Line Results from FREEDOM-HF Study
-
2022

FUROSCIX Approval and Financing
- Resubmission of FUROSCIX® (furosemide injection) New Drug Application
- Positive Results from the AT HOME-HF Phase 2 Pilot Study in Heart Failure
- FDA Approval of FUROSCIX® (furosemide injection)
- $100 Million Debt Financing Agreement with Oaktree
-
2023

FUROSCIX Launch
- Launch and Commercial Availability of FUROSCIX® (furosemide injection)
- Positive Type C Meeting Feedback from FDA on Potential Heart Failure Class IV Indication Expansion
-
2024

Indication Expansion Progress
- First Participant Enrolled in Pivotal Pharmacokinetic Study of FUROSCIX Auto-Injector (furosemide 80mg/mL) Injection
- Filing Acceptance of Supplemental New Drug Application (sNDA) Seeking to Expand FUROSCIX Indication to Include Chronic Kidney Disease
- Positive Topline Study Results for SCP-111 (Furosemide 80 mg/1 mL) Autoinjector
- FDA Approval of Supplemental New Drug Application Expanding the FUROSCIX Indication in Heart Failure
- Financings Totaling Up to $125 Million with Perceptive Advisors
-
2025

Continued FUROSCIX Growth
- Chronic Kidney Disease indication FDA approved on March 6, 2025
Investing in Our
Shared Future
Discover the latest insights into our company’s growth and future vision. Stay informed about the strategic decisions shaping our path forward.
Quick IR Links

Quick IR Links
Newsroom
August 07, 2025
scPharmaceuticals Inc. Reports Second Quarter 2025 Financial Results and Provides Business Update
Generated net FUROSCIX ® revenue of $16 million in the second quarter of 2025; up 99% over Q2 2024 Doses shipped to patients increased 45% over Q1 2025 and 117% over Q2 2024 Launch of FUROSCIX in chronic kidney disease Autoinjector remains on track for sNDA submission in Q3 2025; designed to reduce...
Learn More »July 31, 2025
scPharmaceuticals to Announce Second Quarter 2025 Financial Results After the Market Close on August 7, 2025
Management to host conference call and webcast, after-market on August 7, 2025, at 4:30 p.m. ET BURLINGTON, Mass. , July 31, 2025 (GLOBE NEWSWIRE) -- scPharmaceuticals Inc. (Nasdaq: SCPH), a pharmaceutical company committed to revolutionizing cardiorenal healthcare through patient-centric...
Learn More »May 14, 2025
scPharmaceuticals Inc. Reports First Quarter 2025 Financial Results and Provides Business Update
Generated net FUROSCIX ® revenue of $11.8 million in the first quarter of 2025 Formally launched FUROSCIX in second approved indication, Chronic Kidney Disease, in April 2025 Autoinjector on track for sNDA submission in Q3 2025; designed to reduce treatment time from five hours to less than ten...
Learn More »May 07, 2025
scPharmaceuticals to Announce First Quarter 2025 Financial Results After the Market Close on May 14, 2025
Management to host conference call and webcast, after-market on May 14, 2025, at 4:30 p.m. ET BURLINGTON, Mass. , May 07, 2025 (GLOBE NEWSWIRE) -- scPharmaceuticals Inc. (Nasdaq: SCPH), a pharmaceutical company committed to revolutionizing cardiorenal healthcare through patient-centric innovations,...
Learn More »Empowering Heros on
Career Journeys
scPharmaceuticals is committed to revolutionizing cardiovascular and renal care through patient-centric approaches tailored to enhance outcomes, reduce burden, and increase quality of life.